The Victorian clinical and medical research sector adheres to the highest level of International standards of clinical research.
Single ethical review for clinical trials in Victoria – saving you time within good governance.
Victoria aligns to the Australian Federal Government’s National Approach to Single Ethical Review, whereby one Human Research Ethics Committee (HREC) provides the ethics review for a clinical research proposal that is accepted by other institutions participating in multi-centre clinical trial research in Australia. This ensures your clinical trial benefits from:
- Efficiency – agreed timeframes for processes and procedures are adopted in all jurisdictional systems.
- Trust – the single ethics review of an Australian multi-centre clinical research proposal is accepted by institutions without re-review by their institutional HREC.
- Respect – the National Approach accommodates the differences in jurisdictional statutory and administrative frameworks and institutional arrangements for Australian clinical research sites.
- Compliance – single ethics review of multi-centre human clinical research in Australia meets the requirements of the National Statement to protect human research participants.
The Australian Government’s National Health and Medical Research Council (NHMRC) has certified the ethical review processes of 45 institutions, representing 49 HRECs. Many of these, because of their expertise, have been certified to assess applications for clinical trials in Australia.
How are clinical trials regulated in Victoria?
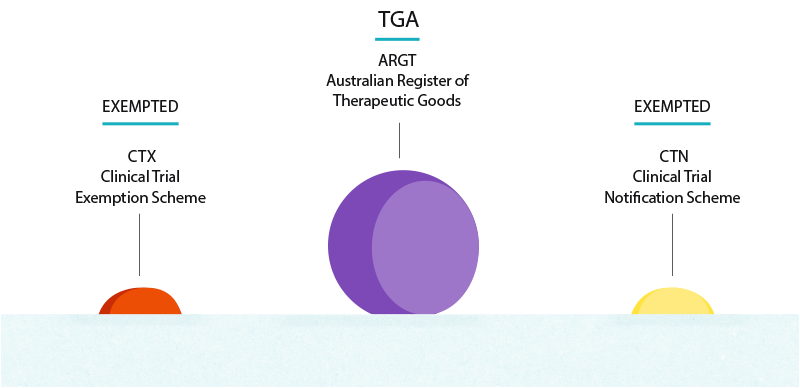
Clinical Trials in Australia are regulated at a number of levels under both Commonwealth, state and territory legislation. The Therapeutic Goods Administration (TGA), a division of the Commonwealth Department of Health, is responsible for regulating medicines and medical devices in a similar way to the Federal Drug Administration (FDA) in the United States.
The TGA oversees the inclusion of medicines and medical devices on the Australian Register of Therapeutic Goods (ARTG). Generally, medicines or medical devices for human use that are imported, manufactured in Australia, supplied by a corporation, supplied interstate or to the Commonwealth, or exported must be included in the ARTG, unless exempted. Unapproved medicines and medical devices to be supplied in a Victorian clinical trial would require an exemption under the Clinical Trial Notification Scheme (CTN) or through the Clinical Trial Exemption Scheme (CTX).
Therapeutic Goods Administration (TGA) is responsible for regulating medicines and medical devices.
Unless under an exempted scheme, they are responsible for your inclusions on the Australian Register of Therapeutic Goods (ARTG).
How are regulatory burdens minimised for clinical trials in Australia?
According to the Australian Trade Commission (Austrade) report, for over two decades Australia’s Clinical Trial Notification (CTN) scheme has been a global benchmark for best practice in reducing the regulatory burden on clinical trial sponsors. The CTN scheme eliminates unnecessary duplication and saves sponsors conducting clinical studies in Australia a significant amount of time and money.
The majority of commercially sponsored clinical trials conducted in Australia are performed under the CTN scheme. All materials relating to a proposed clinical trial in Australia, including the trial protocol, are submitted directly to institutional ethics committees by researchers at the request of the relevant sponsor. The ethics committee is solely responsible for assessing the scientific validity of the trial design, the safety and efficacy of the medicine or device, the ethical acceptability of the trial process, and for the approval of the trial protocol.
The institution at which an Australian clinical trial will be conducted gives the final approval for the conduct of the trial at the site. Under the CTN scheme, the TGA is simply notified of a clinical trial after it has received site approval and does not review any data relating to it. The TGA does have the authority to audit and enquire into the management of a clinical trial.
People who read this were also interested in:
> What are the attractive tax incentives for Australian clinical trials and who is eligible?
> How is valuable intellectual property protected?


